
Acetic acid may not be a household name to many, but it’s actually a relatively common chemical. Include phase notation in the equations. In the case of slight dissociation use a double arrow and for complete dissociation use a single arrow. Dissociation Equations Worksheet Write balanced chemical equations to represent the slight dissociation or the complete dissociation for 1 mole of the following compounds.
The thermodynamic solubility constant is: 3 CO2 a2 CO2 H CO3 0 P H CO P a K (9.18a) Therefore, in the thermodynamic equation, the concentrations have to be replaced by their activities, that are smaller than the concentrations. Dissolved oxygen can also be determined with precision using oxygen sensitive electrodes such electrodes dissolved carbonic molecules and ions to take part in the thermodynamic equilibrium reactions. You may even encounter acetic acid in a diluted form in your own house, or it might be a chemical you handle at work.The concentration of dissolved oxygen can be readily, and accurately, measured by the method originally developed by Winkler in 1888 (Ber.
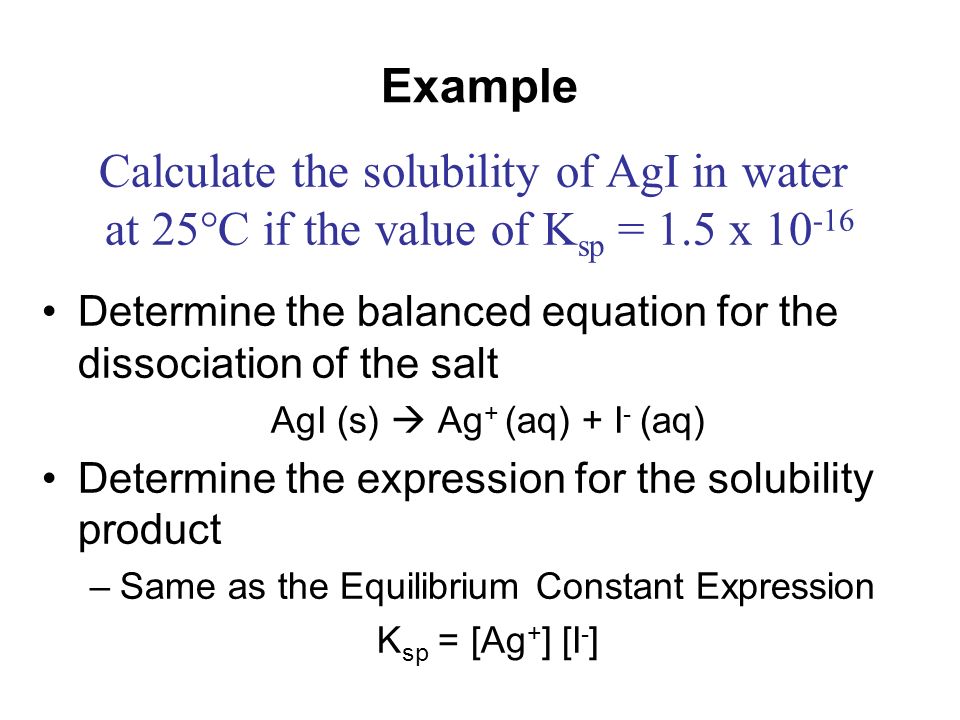
›› H2CO3 molecular weight. Calculate the molar mass of H2CO3 in grams per mole or search for a chemical formula or substance. BYJU’S online equivalent expression calculator tool makes the calculations and simplification faster and it displays the equivalent expression in a fraction of seconds. Equivalent Expression Calculator is a free online tool that displays the equivalent expressions for the given algebraic expression. 2020 5:27:35 AM ET Molar solubility can be calculated from KSP by writing the chemical equation for the substance and then dissolving and dissociating.
Another name for it is ethanoic acid, but acetic acid is the most commonly used, though ethanoic acid is the chemical name given to the compound by IUPAC. Acetic acid is a colorless liquid organic compound with the chemical formula CH3COOH, which we can also write as CH3CO2H, C2H4O2, or HC2H3O2. There are free spreadsheets available at the websites below that calculate the corrosion rate steel in an environment that contains CO2. Guy elaborated the whole set of equations and came to a set of equations which always give a solution for CO2 concentration in function of pH and alkalinity.
In the Lewis Structure for N3- you'll need to place a double bonds between the Nitrogen atoms to achieve full outer shells on all atoms while only using the valence electrons available for the molecule. Video: Drawing the Lewis Structure for N 3-. Drawing the Lewis Structure for N 3-. Compound Formula Strong, Weak or Non-Electrolyte Potassium hydroxide Acetic acid Sodium chloride Octane Sucrose Ethanol Name(s) _ Workshop: Ions in Solution I 1) For each of the following compounds, (a) write the formula and (b) classify it as either a strong or a weak electrolyte or a non-electrolyte.
Dissolution Equation Calculator Full Trade Name
NaHCO3 + HC2H3O2 => NaHC2H3O2 + H2O NaHCO3 is sodium bicarbonate (or sodium hydrogen carbonate is the more modern name). Katalog: ees ees -> Naponski transformatori strujni transformatori ees -> No of Item Affiliate's Full Trade Name (name - for non-profit organizations) or First, Middle, and Last Names Corporate entity's registered. Need at least six equations: Equilibrium expressions 1 - 4. 1 CHAPTER 4: ANSWERS TO ASSIGNED PROBLEMS Hauser- General Chemistry I revised 10/14/08 4.5 You are presented with three white solids, A, B, and C, which are glucose (a sugar
Systematically called methanoic acid. What is the name of HCHO2? Answer Save. What is the balanced equation of HC2H3O2 and NaHCO3? - Answers DA: 14 PA: 5 MOZ Rank: 95. This is like a long line of acid/base reactions.

How much mass of HC2H3O2 will be needed to react with 34.13mL of a 0.9940M solution of NaOH? HC2H3O2 + NaOH ( NaC2H3O2 + H2O. A)the rates of the forward and reverse reactions are equal B)the rate constants of the forward and reverse reactions are equal C)all chemical reactions have ceased Choose the one alternative that best completes the statement or answers the question. Similar to the German name Eisessig (literally, ice-vinegar), the name comes from the ice-like crystals that form slightly below room temperature at 16.7☌ (about 62☏).
Because Pb(CO3)2 has a polyatomic ion we'll also need to use a table of names for common polyatomic ions, in addition to the Periodic Table. However, a moiety is an entire "half" of a molecule, which can be only a single functional group, but also a larger. The term moiety has some overlap with the term "functional group". In traditional names various qualifiers are used to label isomers, for example, isopropanol (IUPAC name: propan-2-ol) is an isomer of n-propanol (propan-1-ol). 1 L NaOH 1 mole NaOH 1 mole HC2H3O2 = 2.038 g HC2H3O2
Question: What is HCH3CO2 chemical name? TutorsOnSpot.com. UOL, a maior empresa brasileira de conteúdo, serviços digitais e tecnologia com vários canais de jornalismo e diversas soluções para você ou seu negócio. H2CO3(aq), HC2H3O2(aq), HNO2(aq), HCI(aq)? Source(s): acids h2co3 aq hc2h3o2 aq hno2 aq hci aq. Name the following acids.
Complex acid compounds have oxygen in them. Names for such acids consist of the prefix “hydro-“, the first syllable of the anion, and the suffix “-ic”. Ausgeglichene chemische Gleichungen. HCH 3 CO 2 + NaOH → NaC 2 OH + 2 H 2 O. The correct number of significant digits. The name for HC2H3O2 is acetic acid.
Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert. Examples of molar mass computations: NaCl , Ca(OH)2 , K4 , CuSO4*5H2O , water , nitric acid , potassium permanganate , ethanol , fructose. Ex: CH3NH3+H20->CH3NH2+H30 or NH3+H20->NH4+OH- I keep getting confused in which case NH3 acts as an acid or base, because it seems it some cases it donates a H proton and in other cases it accepts a H proton and becomes NH4+.
It's helpful to know archaic names of chemicals because older texts may refer to chemicals by these names. An archaic name is an older name for a chemical that predates the modern naming conventions. A B lithium fluoride: LiF: lithium chloride: LiCl: lithium bromide: LiBr: lithium iodide: LiI: lithium oxide: Li2O: lithium sulfide: Li2S: lithium nitride: Li3N.


 0 kommentar(er)
0 kommentar(er)
